Families and patients affected by muscular dystrophy packed the FDA committee meeting, often applauding comments from Sarepta scientists while openly rebuking FDA regulators. More than 50 speakers addressed the FDA during a public comment period that stretched on for hours and included patients, physicians, politicians and even several parents from the United Kingdom who said they would relocate their families to the U.S. if Sarepta's drug is approved here.
Panelists acknowledged the anecdotes, including several teenage boys who said the drug helped them maintain their strength, though a majority of experts said those results were not reflected in Sarepta's data.
"Unfortunately, what I would consider meaningful evidence from the testimony of the families is not properly measured in the study," said Dr. Chiadi Onyike of Johns Hopkins University.
Eteplirsen is an injectable drug intended to treat a subset of patients with Duchenne muscular dystrophy, a fatal genetic disease that causes increasing muscle weakness and eventually the loss of basic movement. The disease affects one of every 3,600 boys worldwide and usually causes death by age 25, according to the National Institutes of Health. There are no drugs that treat the underlying disease, though steroid drugs can slow the loss of muscle strength.
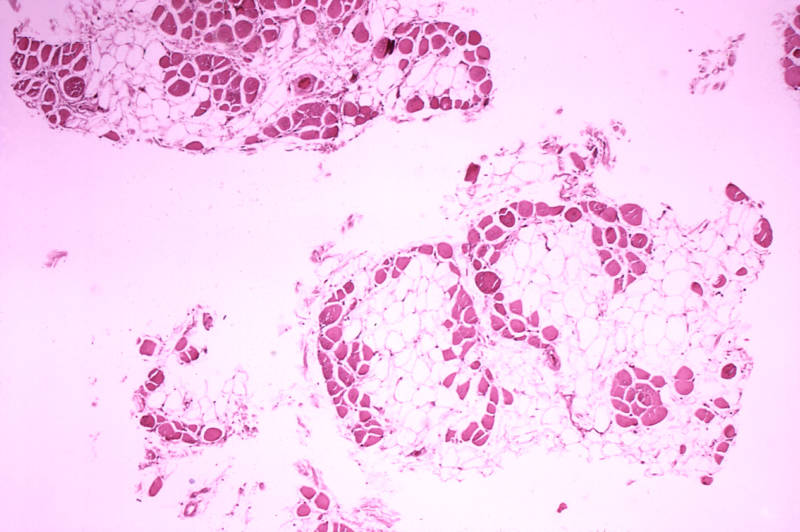
Sarepta's drug is thought to produce a functional protein called dystrophin, which plays a role in muscle fibers.
However, the FDA found numerous problems with the company's study that made it difficult to determine how much dystrophin the drug actually produces, and what, if any, benefit that gives to patients. The company's primary study included just 12 patients and appeared to show an increase in dystrophin of less than 1 percent.
But because of how the trial was structured, the FDA said, comparing study participants to typical muscular dystrophy patients would be an "apples to oranges" comparison. Moreover, regulators said the study showed no significant improvement on its primary goal: performance on a six-minute walking test.
FDA officials showed an unusual degree of candor and emotion over the course of the nearly 12-hour meeting, even addressing the audience directly — something extremely rare within the confines of federal meetings.
At one point, FDA Deputy Division Director Eric Bastings told audience members that he understood their fight, noting that his own family sought out experimental treatments for a sister who was stricken with a rare illness. But he said that experience could not cloud his judgment as a medical reviewer.
"My role, regardless of the pressure that has been placed on my division, is to present scientific conclusions," Bastings said. "We are a science-based organization."
FDA staff emphasized that the agency "strongly encouraged" Sarepta to conduct a larger, more comprehensive study of its drug with a randomly selected control group of patients receiving a placebo — considered the gold-standard of study design.
The FDA is scheduled to make a decision on whether to approve Sarepta's drug by May 26.