It was reported on Monday that the University of Oxford developed a coronavirus vaccine that appears safe and triggers an immune response. Another study from King’s College London from the previous week, however, seems to show that immunity to COVID-19 may only last a few months. And the science of developing a vaccine is only one of many hurdles. Once a vaccine is deemed safe and effective, there are many unanswered questions about how to manufacture and fairly distribute it. In this hour, we talk with a panel of experts about where we are in developing a coronavirus vaccine and how a vaccine should be distributed.
Developing and Distributing a Coronavirus Vaccine
52:46
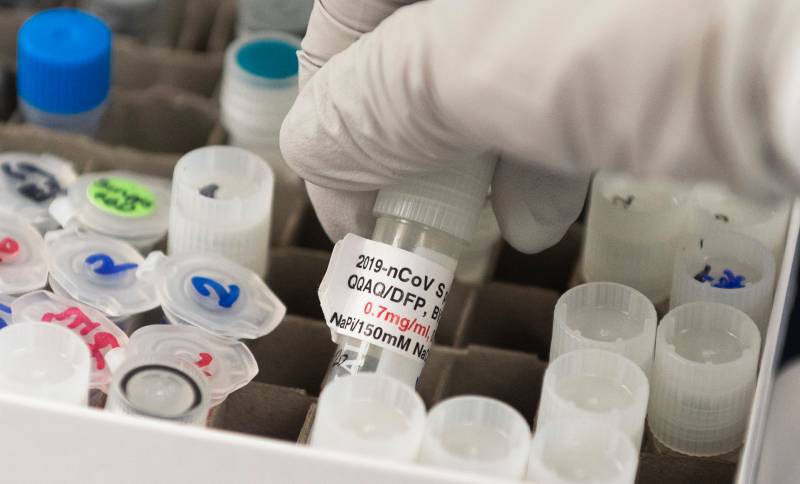
Dr. Nita Patel, Director of Antibody discovery and Vaccine development, lifts a vial with a potential coronavirus, COVID-19, vaccine at Novavax labs in Gaithersburg, Maryland on March 20, 2020. (ANDREW CABALLERO-REYNOLDS/AFP via Getty Images)
Guests:
Dayna Bowen Matthew, Dean and Harold H. Greene Professor of Law, George Washington University Law School; author, "Just Medicine: A Cure for Racial Inequality in American Health Care"; ethics advisor, CDC
Catherine Flores Martin, executive director, California Immunization Coalition
Damian Garde, national biotech reporter, STAT
Dr. Paul Offit, professor of pediatrics, University of Pennsylvania School of Medicine; director, Vaccine Education Center at the Children's Hospital of Philadelphia; author, "Overkill: When Modern Medicine Goes Too Far"
Sponsored